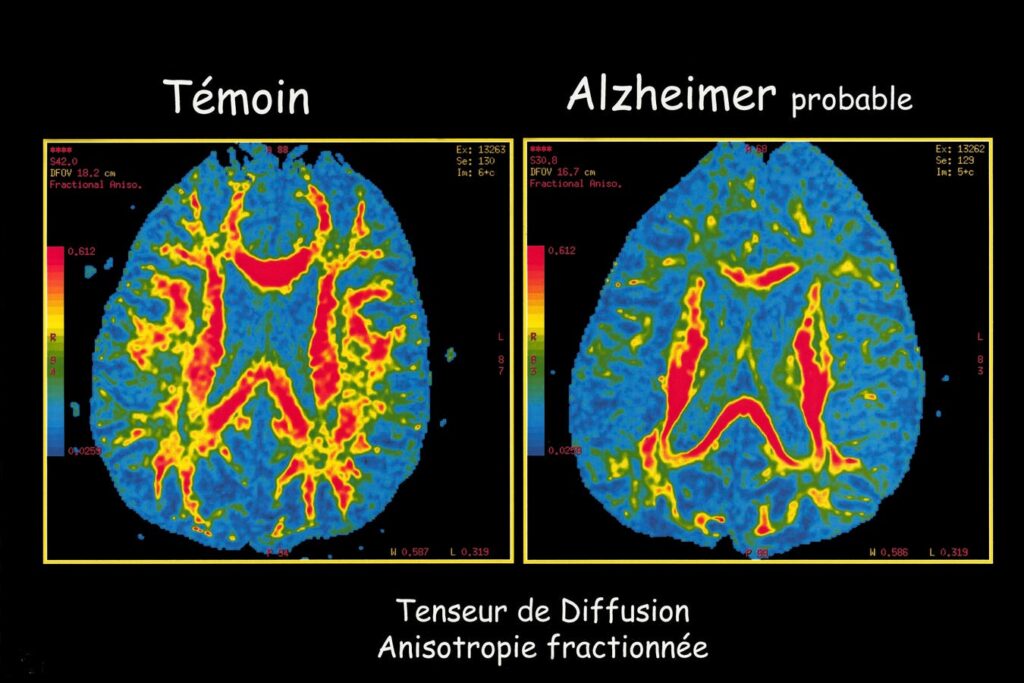
The National Institutes of Health’s Institute of Aging has dropped its sponsorship with the Alzheimer’s Association of a controversial project to revise clinical research standards, which it calls criteria, for determining who has Alzheimer’s disease.
The relationship was unusual. NIH rarely sponsors projects to develop such standards, and almost never partners with advocacy groups. Typically, such guidance is written by expert panels organized by specialty medical associations, such as the American Academy of Neurology.
Eliezer Masliah, director of NIA’s Division of Neuroscience, told me that while NIA still supports efforts to develop guidance for Alzheimer’s research and clinical practice, it does not endorse these criteria. “We are not in the business [saying] one way or the other,” he said.
While NIA partnered in earlier efforts to develop diagnostic standards, its parent NIH ordered NIA to drop its co-sponsorship of the current project. In an email, an NIH spokesman told me, “NIH has determined that because NIA served in an advisory role, the NIA name will be removed from the formal title of the criteria.” NIA staff will continue to work as advisors to the project.
Controversy
The effort has become extremely controversial for two reasons. Its draft says patients may be diagnosed with the disease even though they have no symptoms and may never develop them. And the majority of the members of the expert panel that wrote the recommendations received direct or indirect funding from the Alzheimer’s Association or the drug industry, which stand to benefit from the guidance.
The draft would update a research framework from 2018 and clinical guidelines from 2011. They establish stages of the disease, much like cancer. It says patients with no symptoms can be diagnosed with Alzheimer’s based entirely on biomarkers, such as the amount of proteins called amyloid beta and tau in their brains.
Benefits Of Early Diagnosis
This is unusual, though not unprecedented. For example, anti-cholesterol drugs were developed for people who were pre-symptomatic but showed certain biomarkers in blood tests. The difference is that the current pre-clinical measures of Alzheimer’s are imperfect and can result in people who may never develop symptoms being diagnosed with the disease.
Supporters say early diagnosis can accelerate effective treatment before patients begin to show symptoms such as memory loss. It also can make it easier to enroll asymptomatic patients in drug trials which, they say, can help physicians better understand how these interventions affect those who have no or very mild symptoms.
Downsides
A big change from early guidance is that tests can now accurately measure the amount of amyloid beta and tau in a patient’s brain. But they cannot predict whether the patient ever will develop symptoms of Alzheimer’s disease. The draft acknowledges these weaknesses in current technology.
Critics also have significant concerns about the effects of an Alzheimer’s diagnosis of those without symptoms on drug trials. Enrolling such people may raise important ethical questions (see here, here, and here). For example, many participants in the trial for the anti-Alzheimer’s drug lecanemab developed brain swelling and bleeding and as many as three died from these side effects.
Would a formal diagnosis of Alzheimer’s in trial participants alleviate these ethical concerns? Should it?
And while the guidance is primarily aimed at clinical trials and not for routine medical treatment, it will affect many patients. That could have significant consequences. For example, consider the emotional toll of a diagnosis of an incurable disease such as Alzheimer’s on those who may never develop symptoms.
Windfalls
Critics argue that diagnosing the disease in asymptomatic patients would make it easier for drug companies to sell the costly product to people who will not benefit. And, by substantially boosting the number of people “with Alzheimer’s disease,” it would increase the clout of advocacy groups such as the Alzheimer’s Association.
Similarly, the push for early diagnosis based on biomarkers could be a windfall for testing companies. Currently, the only reliable tests for high levels of amyloid beta are costly PET scans or invasive spinal taps. But firms already are beginning to market home tests at a cost of $399, even though the Food and Drug Administration has not certified their reliability.
Conflicts
There also are questions about the membership of the expert panel that is developing the guidance. Of the 22 members, 16 either work for or have received consulting fees or research grants from drugmakers or the Alzheimer’s Association, which itself is heavily funded by the drug industry. Only two independent researchers participated, and no representatives of people living with Alzheimer’s, their families, or consumer groups. The Alzheimer’s Association has lobbied aggressively for FDA approval and for Medicare payments for anti-Alzheimer’s drugs.
The draft standards explicitly state that treating physicians should use their best judgement in diagnosing Alzheimer’s and add, “These new criteria do not constitute clinical practice guideline recommendations.” Still, they remain extremely controversial and inevitably will affect the way doctors treat their patients. And that may help explain why NIH removed its sponsorship of the project.
Read the full article here