People would rather live in a community with unreasonable claims, than face loneliness with their truth”― Bangambiki Habyarimana
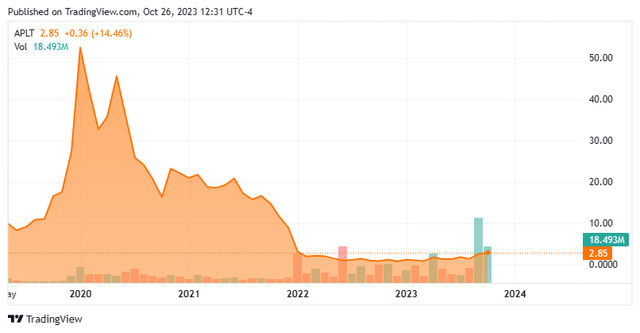
Today, we put Applied Therapeutics, Inc. (NASDAQ:APLT) in the spotlight for the first time. The company is on the cusp of filing its first marketing application to the FDA. The stock is also up some 300% in trading since late April. Can the rally in this small biotech developmental company continue? An analysis follows below.
Seeking Alpha
Company Overview:
This clinical stage biotech concern is headquartered in New York City. The company is focused on developing novel products to target central nervous system rare diseases and diabetic complications. The stock currently trades just south of three bucks a share and sports an approximate market capitalization of $175 million.
The company is hoping to speed its time to market within its pipeline by targeting molecules and pathways whose roles in disease development are already proven and using technology as well as science to design products with increased potency and selectivity.
Company Website
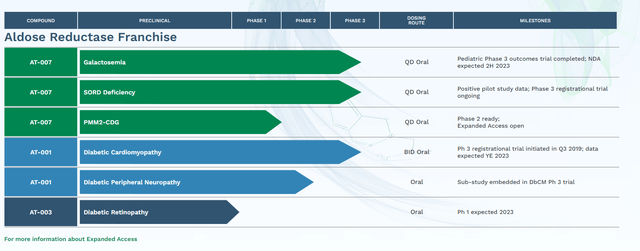
The company has two main candidates within its pipeline at the moment. They are AT-001 and AT-007 (also known as govorestat) which are both Aldose Reductase Inhibitors or ARIs. According to Wikipedia, Aldose Reductase is:
An enzyme that is normally present in many other parts of the body and catalyzes one of the steps in the sorbitol (polyol) pathway that is responsible for fructose formation from glucose. Aldose reductase activity increases as the glucose concentration rises in diabetes in those tissues that are not insulin sensitive, which include the lenses, peripheral nerves, and glomerulus. Sorbitol does not diffuse through cell membranes easily and therefore accumulates, causing osmotic damage which leads to retinopathy and neuropathy.“
AT-007 is being developed for a rare metabolic disorder called Galactosemia. The company disclosed in early September that it had conducted a ‘Pre-NDA meeting’ with the FDA. A marketing application for this indication should be filed before year-end. That announcement was the primary trigger behind the huge rise of the stock over the past two months. A Phase 3 trial was completed this spring with the following results.
AT-007 is also being evaluated to treat Sorbitol Dehydrogenase Deficiency or SORD. The candidate showed encouraging results in a pilot study and currently is being evaluated in an ongoing Phase 3 registrational trial ‘INSPIRE’. AT-007 has Orphan Drug Designation for both Galactosemia and SORD as well as Pediatric Rare Disease and Fast Track designations for the former indication. An August Seeking Alpha article did a good job of highlighting the potential markets for both Galactosemia and SORD.

DbCM (Company Website)
The company’s second primary ARI is AT-001 which currently is focusing on treating Diabetic Cardiomyopathy (DbCM) as well as Diabetic Peripheral Neuropathy. A registrational study for AT-001 to treat DbCM was initiated originally in 2019. Data from that trial should be disclosed sometime before the end of this year.
Analyst Commentary & Balance Sheet:
Only Robert W. Baird has offered up a rating among analyst firms around Applied Therapeutics, Inc. in 2023. They reiterated their Buy rating and $14 price target on the stock last month.
Approximately five percent of the outstanding float in the shares are currently held short. Three insiders sold approximately $360,000 worth of shares collectively near the end of September. It was the first notable insider transaction in the stock in 2023. The company ended the second quarter with just over $35 million in cash and marketable securities on its balance sheet after posting a net loss of $29.6 million for the quarter. The company carries no long-term debt.
Verdict:
The biggest issue in chasing the recent rally in APLT is that the company is going to have to do a capital raise in the very near future. Given the firm’s current burn rate and market capitalization, that event is likely to be quite dilutive. Applied Therapeutics filed a $300 million mixed shelf offering in late May of this year it should be noted.
In addition, the stock has evaporated a ton of shareholder value overall since it peaked early in 2020. Therefore, the recommendation is to ‘avoid‘ until the capital raise is done and the market application has been submitted and then to re-evaluate the investment thesis at what are likely to be lower potential entry points.
Just because an idea is true doesn’t mean it can be proved. And just because an idea can be proved doesn’t mean it’s true.”― Jonah Lehrer
Read the full article here